Diasmoke

Smoking and diabetes
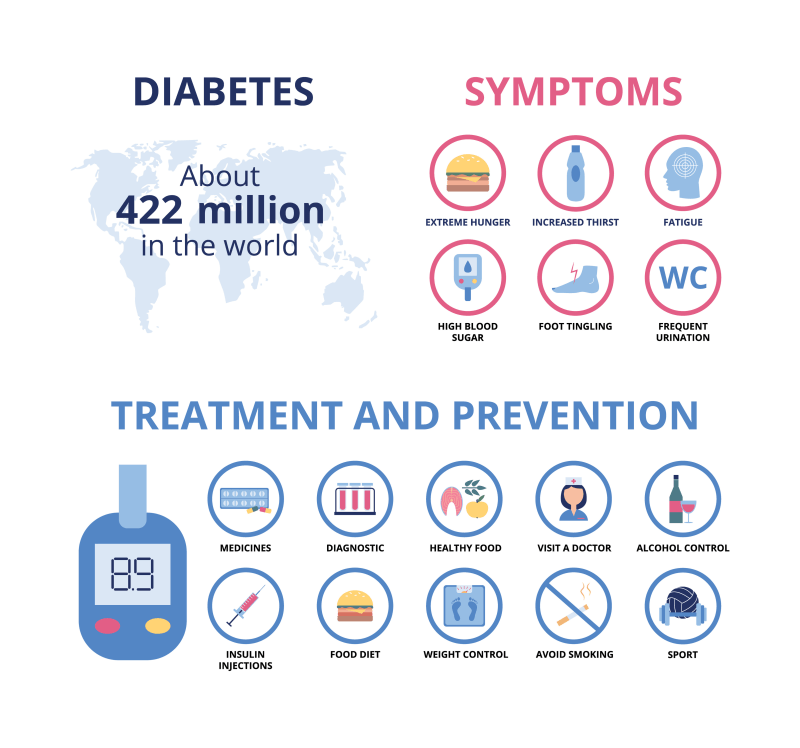
Living with diabetes means facing the choice of a healthy lifestyle every day. Having diabetes means having a disease that often raises blood sugar levels in a worrying way. There are several types of diabetes. Type 2 is the most common in adults and accounts for over 90% of all diabetes cases. Fewer people have type 1 diabetes, which more often develops in children, adolescents, or young adults.
Smoking and diabetes are a bad combination. Regardless of the type of diabetes you have, smoking makes your diabetes harder to control. If you have diabetes and smoke, you are more likely to have serious diabetes-related health problems. Smokers with diabetes have a higher risk of serious complications.
What is Diasmoke
Little is known about the impact of combustion-free nicotine delivery sistem on type 2 diabetes mellitus (T2DM) patients who smoke. DIASMOKE (Assessing the impact of combustion free-nicotine delivery technologies in Diabetic Smokers) is an international, multicenter, open label randomized controlled study designed to determine wheter T2DM cigarette smokers switching to CF-NDS experience measurable improvement in cardiovascular risk parameters as a consequence of avoiding exposure to cigarette smoke toxicants.
This study aims to test the hypothesis that avoiding exposure to cigarette smoke toxicants may translate to measurable improvement in cardiovascular risk factors and functional parameters when T2DM patients who smoke switch to using C-F NDS compared with T2DM patients who continue to smoke conventional tobacco products.
Your health will be monitored and monitored continuously.
What Does the Study Involve?
The study involves recruiting a total 576 patients will be randomized to either a control arm, in which they will be offered referral to smoking cessation programs or to an intervention arm, in which they will be assigned to C-F NDS use.
What is the Aim of Diasmoke?
Aim of Diasmoke is to determine whether type 2 diabetes mellitus cigarettes smokers whose switch to combustion free nicotine delivery system experience measurable improvements in cardiovascular risk parameters
Why participate in Diasmoke
What is the purpose of the study?
This study investigates whether vaping or using E-cigarettes reduces the risk of cardiovascular (heart and circulation) disease as compared to normal smoking. The results of the study may help us to understand more about the risks of vaping or e-cigarettes and normal cigarettes for diabetes patients who are smokers.
What do we ask you?
Before randomization, all smokers will be reminded of the risks associated with smoking and will be offered a free smoking cessation program according to standard local guidelines and depending on the local availability of antismoking services. Everyone who joins the study will be randomly chosen to be in one of two groups of participants. One group will be asked to carry on smoking their usual cigarettes for the duration of the study, and the second group will be asked to switch to vaping.
Duration
The study will last approximately 2 years in total. After the basic checkup, you will be asked to come back for another four checkups after 3 months, 6 months, one year and two years.
DiasmokeFree Working Group
In 2023, the Diasmoke research team set up an International Working Group with the world’s leading experts in diabetes to address the issue of smoking harm in people with diabetes, in its clinical, medical, behavioral and sociological implications, in order to provide independent consultation and oversight to the development of smoking cessation / harm reduction guidelines for smokers with type 2 diabetes mellitus.
Center of Excellence for the Acceleration of HArm Reduction [CoEHAR], University of Catania, Catania, Italy; Centre for the Prevention and Treatment of Tobacco Addiction [CPCT], Catania, Italy; Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Clinica Medica “A. Murri,” Department of Precision and Regenerative Medicine and Ionian Area [DiMePre-J], University “Aldo Moro” Medical School, Bari, Italy
Royal Cornwall Hospital NHS Trust, Treliske, Truro, U.K.
Non-Communicable Diseases Research Unit, South African Medical Research Council and University of Cape Town, Cape Town, South Africa; Department of Biological and Environmental Science, Faculty of Science, Walter Sisulu University, Mthatha, South Africa
Diabetes Foundation [India], New Delhi, India; National Diabetes, Obesity and Cholesterol Foundation [N-DOC], New Delhi, India; Fortis C-DOC Centre for Excellence for Diabetes, Metabolic Disease, and Endocrinology, New Delhi, India
Shaukat Khanum Cancer Hospital and Research Center, Peshawar, Pakistan
Ashford and Saint Peter’s Hospitals NHS Foundation Trust, Chertsey, U.K.
Faculty of Health Sciences, University of Malta, Valletta, Malta; id-Dar tal-Providenza, Siggiewi, Malta
Diabetes and Hormone Center, Colombo, Sri Lanka; South Asian Federation of Endocrine Societies, Colombo, Sri Lanka; National Hospital of Sri Lanka, Colombo, Sri Lanka; Endocrine Society of Sri Lanka, Colombo, Sri Lanka
Department of Human Epigenetics, Mossakowski Medical Research Institute, Polish Academy of Sciences, Warsaw, Poland; Department of Internal Diseases, Endocrinology, and Diabetology, The National Institute of Medicine of the Ministry of Interior and Administration, Warsaw, Poland
Press
At GFN 2023, SAB Diasmoke second meeting
Diasmoke 2.0: how to participate to the project?
CoEHAR is the promoter and coordinator
of the Diasmoke Study

CoEHAR is a unique multidisciplinary research center focused on the study of Tobacco Harm Reduction. Innovation, peer-review research, and globalization of ideas are at the core of CoEHAR actions.
Established in 2018 at the University of Catania, CoEHAR has a large network of international collaborations with experts from different fields and countries. CoEHAR mission is to create a new global, shared and controlled science that can improve its applications with specific solutions for the needs of each country and territory.
Partners

Mossakowski Clinical Research Centre
Warsaw, Poland

Mossakowski Clinical Research Centre
Warsaw, Poland
Mossakowski Clinical Research Centre is a part of Polish Academy of Sciences scientific structure.
As most of its units are theoretical ones, it cooperates with Central Clinical Hospital MSWiA in Warsaw, one of the biggest hospitals in Poland, performing together many clinical and academic studies.
The DiASMOKE study will be performed in the Diabetology Center of the hospital, an ambulatory unit supervising more than 4000 diabetic patients.
PI: Edward Franek
Investigator: Arkadiusz Krysinski

Duo Clinic
Chisinau, Moldova

Duo Clinic
Chisinau, Moldova
PI: Dr. Igor Ivanes

ARNAS Garibaldi Hospital
Catania, Italy

ARNAS Garibaldi Hospital
Catania, Italy
PI: Dr. Igor Ivanes

AOUP University Hospital
Catania, Italy

AOUP University Hospital
Catania, Italy
PI: Dr. Igor Ivanes

University of Palermo
Palermo, Italy

University of Palermo
Palermo, Italy
PI: Dr. Igor Ivanes

University of Bari
Bari, Italy

University of Bari
Bari, Italy
PI: Dr. Igor Ivanes

Ashford and St Peter's Hospitals NHS Foundation Trust
Ashford and Chertsey, UK

Metanoic Health Ltd
New Malden, Surrey, England
Timeline
Apply to be a study subject
Download our Media Kit
Lorem Ipsum Dolor sit amet